Abstract
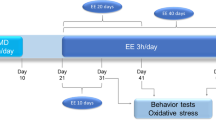
Both prenatal ethanol and early-life stress have been shown to induce reduced risk-taking and explorative behavior as well as cognitive dysfunction in the offspring. In this study, we examined the effect of combined exposure to ethanol and early stress on maternal care, exploratory behavior, memory performances, and oxidative stress in male offspring. Pregnant rats were exposed to ethanol (4 g/kg) from gestational day (GD) 6–to postnatal day (PND) 14 and limited nesting material (LNS) from PND0-PND14 individually or in combination. Maternal behavior was evaluated during diurnal cycle. The level of corticosterone hormone and markers of oxidative stress were evaluated in the pups. Risk-taking and explorative behavior were assessed with the elevated-plus maze (EPM) test and cognitive behavior with the Morris water maze (MWM), novel object recognition (NORT), and object location memory (OLM) tests. In the mothers, perinatal alcohol or LNS either alone or in combination decreased maternal behavior. In the offspring, the combination of the two factors significantly increased the pup’s plasma corticosterone concentration in comparison with ethanol and LNS alone. Reduced risk-taking behavior was observed in the ethanol, LNS and ethanol + LNS groups compared with the control group, and this was amplified in the co-exposure of ethanol and LNS groups. The MWM, NORT, and OLM tests revealed spatial and recognition memory impairment in the ethanol and LNS groups. This impairment was more profound in the co-exposure of ethanol and LNS. Also, we observed a significant decrease in superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities and an increase in malondialdehyde (MDA) level in the hippocampus of ethanol and LNS co-exposed animals as compared with individual exposure of ethanol and LNS. While each factor independently produced similar outcomes, the results indicate that the dual exposure paradigm could significantly strengthen the outcomes.










Similar content being viewed by others
References
Aisa B, Elizalde N, Tordera R, Lasheras B, Del Río J, Ramírez MJ (2009a) Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus 19(12):1222–1231
Aisa B, Gil-Bea FJ, Marcos B, Tordera R, Lasheras B, Del Río J, Ramírez MJ (2009b) Neonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: involvement of the HPA axis. Psychoneuroendocrinology 34(10):1495–1505
An L, Yang Z, Zhang T (2013) Imbalanced synaptic plasticity induced spatial cognition impairment in male offspring rats treated with chronic prenatal ethanol exposure. Alcohol Clin Exp Res 37(5):763–770. https://doi.org/10.1111/acer.12040
Avishai-Eliner S, Gilles E, Eghbal-Ahmadi M, Bar-El Y, Baram T (2001) Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol 13(9):799–807
Bagheri F, Goudarzi I, Lashkarbolouki T, Elahdadi Salmani M (2015) Melatonin prevents oxidative damage induced by maternal ethanol administration and reduces homocysteine in the cerebellum of rat pups. Behav Brain Res 287:215–225. https://doi.org/10.1016/j.bbr.2015.03.022
Bassani TB, Turnes JM, Moura ELR, Bonato JM, Coppola-Segovia V, Zanata SM, Oliveira R, Vital M (2017) Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav Brain Res 335:41–54. https://doi.org/10.1016/j.bbr.2017.08.014
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sanchez-Diaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of medicago sativa. Plant Physiol 82(4):1169–1171. https://doi.org/10.1104/pp.82.4.1169
Becker HC (2012) Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research: Current Reviews 34(4):448
Behl C, Trapp T, Skutella T, Holsboer F (1997) Protection against oxidative stress-induced neuronal cell death–a novel role for RU486. Eur J Neurosci 9(5):912–920. https://doi.org/10.1111/j.1460-9568.1997.tb01442.x
Berman RF, Hannigan JH (2000) Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 10(1):94–110. https://doi.org/10.1002/(SICI)1098-1063(2000)10:1%3c94::AID-HIPO11%3e3.0.CO;2-T
Binienda Z, Kim CS (1997) Increase in levels of total free fatty acids in rat brain regions following 3-nitropropionic acid administration. Neurosci Lett 230(3):199–201. https://doi.org/10.1016/s0304-3940(97)00514-4
Borsoi M, Antonio CB, Viana AF, Nardin P, Goncalves CA, Rates SM (2015) Immobility behavior during the forced swim test correlates with BNDF levels in the frontal cortex, but not with cognitive impairments. Physiol Behav 140:79–88. https://doi.org/10.1016/j.physbeh.2014.12.024
Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ (2005) Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci Off J Soc Neurosci 25(41):9328–9338. https://doi.org/10.1523/JNEUROSCI.2281-05.2005
Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ (2005b) Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 25(41):9328–9338
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci 95(9):5335–5340
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301(5631):386–389
Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, De Kloet ER, Joëls M, Krugers H (2008) Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci 28(23):6037–6045
Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E, Choi IA, Lee KS, Shin YJ, Shin YH, Kim YH, Kim KW, Ahn K, Won HS, Choi SJ, Oh SY, Kwon JY, Kim YH, Park HJ, Lee KJ, Jun JK, Yu HS, Lee SH, Jung BK, Kwon JW, Choi YK, Do N, Bae YJ, Kim H, Chang WS, Kim EJ, Lee JK, Hong SJ (2016) Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol 138(2):468–475 e465 https://doi.org/10.1016/j.jaci.2016.01.020
Cohen-Kerem R, Koren G (2003) Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol 25(1):1–9 https://doi.org/10.1016/s0892-0362(02)00324-0
Dalle Molle R, Portella A, Goldani M, Kapczinski F, Leistner-Segala S, Salum G, Manfro G, Silveira P (2012a) Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2(11):e195–e195
Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, Manfro GG, Silveira PP (2012b) Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2:e195. https://doi.org/10.1038/tp.2012.126
Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA (2003) Early life conditions and cognitive functioning in later life. Am J Epidemiol 158(11):1083–1089
Farrokhi E, Samani KG, Chaleshtori MH (2014) Oxidized low-density lipoprotein and upregulated expression of osteonectin and bone sialoprotein in vascular smooth muscle cells. Laboratory Medicine 45(4):297–301
Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C (2005) Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res 30(1):105–111. https://doi.org/10.1007/s11064-004-9691-6
Gatta E, Mairesse J, Deruyter L, Marrocco J, Van Camp G, Bouwalerh H, Lo Guidice JM, Morley-Fletcher S, Nicoletti F, Maccari S (2018) Reduced maternal behavior caused by gestational stress is predictive of life span changes in risk-taking behavior and gene expression due to altering of the stress/anti-stress balance. Neurotoxicology 66:138–149. https://doi.org/10.1016/j.neuro.2018.04.005
Gilles EE, Schultz L, Baram TZ (1996) Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 15(2):114–119
Gomez JL, Lewis MJ, Luine VN (2012) The interaction of chronic restraint stress and voluntary alcohol intake: effects on spatial memory in male rats. Alcohol 46(5):499–504
Gustaw-Rothenberg K, Lerner A, Bonda DJ, Lee HG, Zhu X, Perry G, Smith MA (2010) Biomarkers in Alzheimer’s disease: past, present and future. Biomark Med 4(1):15–26. https://doi.org/10.2217/bmm.09.86
Hellemans KG, Sliwowska JH, Verma P, Weinberg J (2010) Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev 34(6):791–807. https://doi.org/10.1016/j.neubiorev.2009.06.004
Hofer MA (1994) Early relationships as regulators of infant physiology and behavior. Acta Paediatr 83:9–18
Huot RL, Plotsky PM, Lenox RH, McNamara RK (2002) Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 950(1–2):52–63
Ivy AS, Brunson KL, Sandman C, Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154(3):1132–1142. https://doi.org/10.1016/j.neuroscience.2008.04.019
Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ (2010) Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci 30(39):13005–13015
Joëls M (2018) Corticosteroids and the brain. J Endocrinol 238(3):R121–R130
Juruena MF (2014) Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy & Behavior : E&b 38:148–159. https://doi.org/10.1016/j.yebeh.2013.10.020
Karch SB (2007) Addiction and the medical complications of drug abuse. CRC Press
Katyare SS, Pandya JD (2005) A simplified fluorimetric method for corticosterone estimation in rat serum, tissues and mitochondria. Indian J Biochem Biophys 42(1):48–53
Kehoe P, Shoemaker W (1991) Opioid-dependent behaviors in infant rats: effects of prenatal exposure to ethanol. Pharmacol Biochem Behav 39(2):389–394. https://doi.org/10.1016/0091-3057(91)90197-a
Kim EJ, Pellman B, Kim JJ (2015) Stress effects on the hippocampus: a critical review. Learn Mem 22(9):411–416
King S, Laplante DP (2005) The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress 8(1):35–45
Lam VYY, Raineki C, Ellis L, Yu W, Weinberg J (2018) Interactive effects of prenatal alcohol exposure and chronic stress in adulthood on anxiety-like behavior and central stress-related receptor mRNA expression: Sex- and time-dependent effects. Psychoneuroendocrinology 97:8–19. https://doi.org/10.1016/j.psyneuen.2018.06.018
Lee Y, Rowe J, Eskue K, West JR, Maier SE (2008) Alcohol exposure on postnatal day 5 induces Purkinje cell loss and evidence of Purkinje cell degradation in lobule I of rat cerebellum. Alcohol 42(4):295–302. https://doi.org/10.1016/j.alcohol.2008.01.010
Lu Y-L, Richardson HN (2014) Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 277:139–151
Lupien SJ, McEwen BS (1997) The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 24(1):1–27
Mahdinia R, Goudarzi I, Lashkarbolouki T, Salmani ME (2019) Vitamin E attenuates alterations in learning, memory and BDNF levels caused by perinatal ethanol exposure. Nutritional Neuroscience 1–15 https://doi.org/10.1080/1028415X.2019.1674523
Mattson SN, Crocker N, Nguyen TT (2011) Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev 21(2):81–101. https://doi.org/10.1007/s11065-011-9167-9
McEwen BS, Eiland L, Hunter RG, Miller MM (2012) Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62(1):3–12
McIntosh LJ, Cortopassi KM, Sapolsky RM (1998a) Glucocorticoids may alter antioxidant enzyme capacity in the brain: kainic acid studies. Brain Res 791(1–2):215–222. https://doi.org/10.1016/s0006-8993(98)00104-8
McIntosh LJ, Hong KE, Sapolsky RM (1998b) Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res 791(1–2):209–214. https://doi.org/10.1016/s0006-8993(98)00115-2
McIntosh LJ, Sapolsky RM (1996) Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol 141(2):201–206. https://doi.org/10.1006/exnr.1996.0154
McLaughlin KJ, Gomez JL, Baran SE, Conrad CD (2007) The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res 1161:56–64. https://doi.org/10.1016/j.brainres.2007.05.042
Mirescu C, Gould E (2006) Stress and adult neurogenesis. Hippocampus 16(3):233–238
Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M (2014) Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav Brain Res 270:196–205. https://doi.org/10.1016/j.bbr.2014.05.015
Moriceau S, Shionoya K, Jakubs K, Sullivan RM (2009) Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci Off J Soc Neurosci 29(50):15745–15755. https://doi.org/10.1523/JNEUROSCI.4106-09.2009
Morris RG (2008) Morris water maze scholarpedia 3(8):6315
Moussaoui N, Larauche M, Biraud M, Molet J, Million M, Mayer E, Taché Y (2016) Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS One 11(5):e0155037
Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A (2015) Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 25(3):309–328
Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja EL, Karlsson H (2016) Maternal prenatal stress and infant emotional reactivity six months postpartum. J Affect Disord 199:163–170. https://doi.org/10.1016/j.jad.2016.04.020
O’Connor TG, Heron J, Golding J, Glover V, Team ASS (2003) Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 44(7):1025–1036
O’connor MJ, Paley B (2006) The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J Pediatr Psychol 31(1):50–64
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Penza KM, Heim C, Nemeroff CB (2003) Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Womens Ment Health 6(1):15–22. https://doi.org/10.1007/s00737-002-0159-x
Rajput P, Jangra A, Kwatra M, Mishra A, Lahkar M (2017a) Alcohol aggravates stress-induced cognitive deficits and hippocampal neurotoxicity: protective effect of melatonin. Biomed Pharmacother 91:457–466
Rajput P, Jangra A, Kwatra M, Mishra A, Lahkar M (2017b) Alcohol aggravates stress-induced cognitive deficits and hippocampal neurotoxicity: Protective effect of melatonin. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 91:457–466 https://doi.org/10.1016/j.biopha.2017b.04.077
Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM (2017) Stress facilitates the development of cognitive dysfunction after chronic ethanol exposure. Alcohol Clin Exp Res 41(9):1574–1583
Rodriguez A, Bohlin G (2005) Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry 46(3):246–254
Rose A, Shaw S, Prendergast M, Little H (2010) The importance of glucocorticoids in alcoholdependence and neurotoxicity. Alcohol Clin Exp Res 34(12):2011–2018
Russo A, Palumbo M, Scifo C, Cardile V, Barcellona ML, Renis M (2001) Ethanol-induced oxidative stress in rat astrocytes: role of HSP70. Cell Biol Toxicol 17(3):153–168. https://doi.org/10.1023/a:1011936313510
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229
Schneider ML, Moore CF, Kraemer GW (2004) Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child Dev 75(1):96–109
Shirpoor A, Minassian S, Salami S, Khadem-Ansari MH, Ghaderi-Pakdel F, Yeghiazaryan M (2009) Vitamin E protects developing rat hippocampus and cerebellum against ethanol-induced oxidative stress and apoptosis. Food Chem 113(1):115–120
Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, Machado RB, Carvalho RC, Ribeiro Rde A, Tufik S, Frussa-Filho R (2004) Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 46(6):895–903. https://doi.org/10.1016/j.neuropharm.2003.11.032
Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T (2016a) The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: Role of oxidative stress. Food and Chemical Toxicology : an International Journal Published for the British Industrial Biological Research Association 96:263–272. https://doi.org/10.1016/j.fct.2016.07.009
Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T (2016b) The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: Role of oxidative stress. Food Chem Toxicol 96:263–272
Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T (2017) Maternal administration of melatonin prevents spatial learning and memory deficits induced by developmental ethanol and lead co-exposure. Physiol Behav 173:200–208. https://doi.org/10.1016/j.physbeh.2017.02.012
Staples MC, Porch MW, Savage DD (2014) Impact of combined prenatal ethanol and prenatal stress exposures on markers of activity-dependent synaptic plasticity in rat dentate gyrus. Alcohol 48(6):523–532. https://doi.org/10.1016/j.alcohol.2014.06.006
Staples MC, Rosenberg MJ, Allen NA, Porch MW, Savage DD (2013) Impact of combined prenatal ethanol and prenatal stress exposure on anxiety and hippocampal-sensitive learning in adult offspring. Alcohol Clin Exp Res 37(12):2039–2047. https://doi.org/10.1111/acer.12190
Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’malley K, Young JK (2004) Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr 25(4):228–238
Vaglenova J, Pandiella N, Wijayawardhane N, Vaithianathan T, Birru S, Breese C, Suppiramaniam V, Randal C (2008) Aniracetam reversed learning and memory deficits following prenatal ethanol exposure by modulating functions of synaptic AMPA receptors. Neuropsychopharmacology 33(5):1071–1083. https://doi.org/10.1038/sj.npp.1301496
Weinberg J, Petersen TD (1991) Effects of prenatal ethanol exposure on glucocorticoid receptors in rat hippocampus. Alcoholism: Clinical and Experimental Research 15(4):711–716
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65(5):427–451
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333. https://doi.org/10.1016/s0076-6879(81)77046-0
Willoughby KA, Sheard ED, Nash K, Rovet J (2008) Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14(6):1022–1033
Zhang X, Sliwowska JH, Weinberg J (2005) Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med 230(6):376–388. https://doi.org/10.1177/15353702-0323006-05
Acknowledgements
We acknowledge Damghan University for supporting this work.
Author information
Authors and Affiliations
Contributions
F.B. performed all the experiments and wrote the manuscript; I.G. designed research project, wrote the manuscript and contributed to interpretation of the data, T.L., A.F. contributed in biochemical study, interpretation of the data and the manuscript editing, M.E.S. contributed to interpretation of data and the manuscript editing and S.M.F contributed in study design and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagheri, F., Goudarzi, I., Lashkarbolouki, T. et al. The Combined Effects of Perinatal Ethanol and Early-Life Stress on Cognition and Risk-Taking Behavior through Oxidative Stress in Rats. Neurotox Res 40, 925–940 (2022). https://doi.org/10.1007/s12640-022-00506-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00506-6